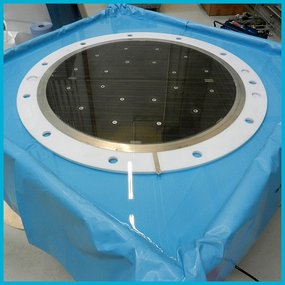
Alkaline Pressure Electrolysis

The generation of hydrogen by alkaline water electrolysis is a key technology for the hydrogen energy cycle. In this video we briefly present the hydrogen research center of the BTU.
With an alkaline pressure electrolysis system and further test rigs, we conduct research on the following topics:
- Component tests
- Investigation of different dynamic modes of operation
- In-operando analysis at single-cell level
- Thermal management (quick start, use of waste heat)
- H2 post-treatment (cleaning, drying, compression)
- Multi-scale simulation models (model verification)
- Digital twin
Technical specifications
- Electrolyzer stack: 24 single cells in bipolar filter-press design
- Electrode area: 0.56 m2
- Operational pressure: 10 ... 58 bar
- Operational temperature: 70 ... 80 °C
- Hydrogen gas production: max. 30 Nm3/h
- Gas purity (after electrolysis):
- H2: 99.9 ± 0.1 vol.%
- O2: 99.5 ± 0.3 vol.%
- H2 purity after cleaning: 99.999 vol.%
- Operating current DC: 2,000 A (max. 3,000 A)
- Nominal power: 95 kW
- Partial and overload range: 5 ...150 %
- Specific energy consumption: approx. 4.5 kWh/Nm³
- Electrolyte: 25 ... 30 mass% KOH
- H2 storage tank: max. 2,000 Nm3 at 45 bar
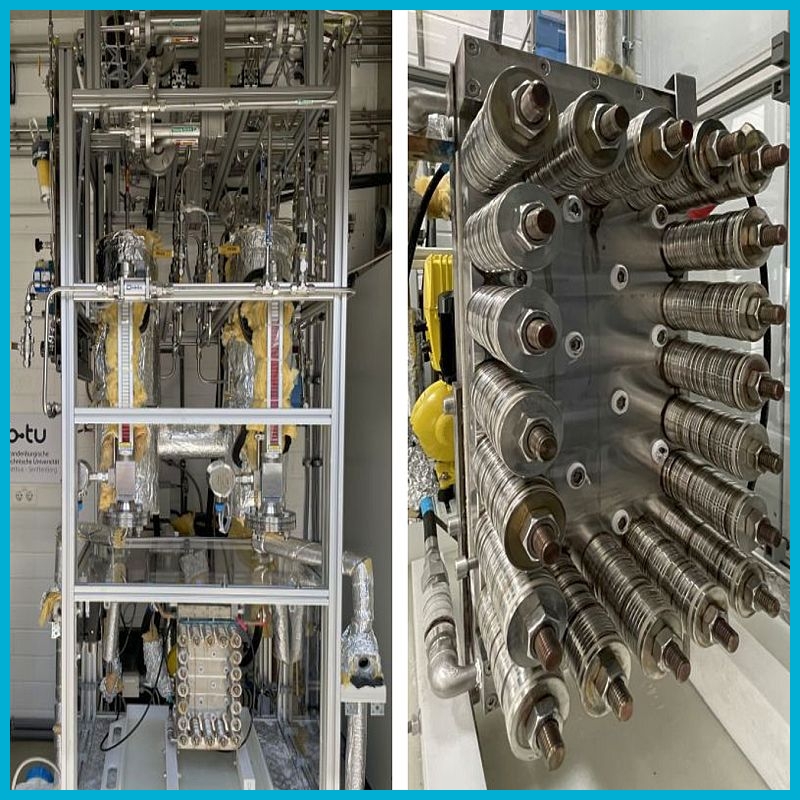
Single-cell test rig
A unique single-cell test rig is used to test cell components such as electrodes or separators in operando or to study the behavior of gas-bubble-electrolyte flow:
- Operational temperature: max. 150 °C
- Operational pressure: max. 23.5 bar
- Electrolyte concentration: max. 30 mass% KOH
- Adjustable volume flow rate in electrolyte circle: max. 15.0 l/min
- Electrolyte feed mode: simultaneous on cathode and anode side
- Hydrogen production rate: up to 250 Nl/h (at 600 A)
- Operating current (DC): up to 600 A (extensible up to 1,200 A)
- Cell material: stainless steel
- Cell active catalyst area: 360 cm2
- Cell flowfield depths: 2.25 ... 31.5 mm
- Distance electrode-separator: quasi zero-gap up to 8 mm
- In-operando investigations:
- Bubble formation and flow (videoscopy)
- Current density distribution on cathode and anode side
- Temperature distribution on cathode and anode side
- Electrochemical impedance spectroscopy and further electrochemical measurement methodes (GAMRY potentiostat & booster)