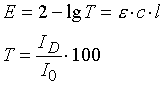Atomic absorption spectrometry
Determination of the exchanged Fe-ions
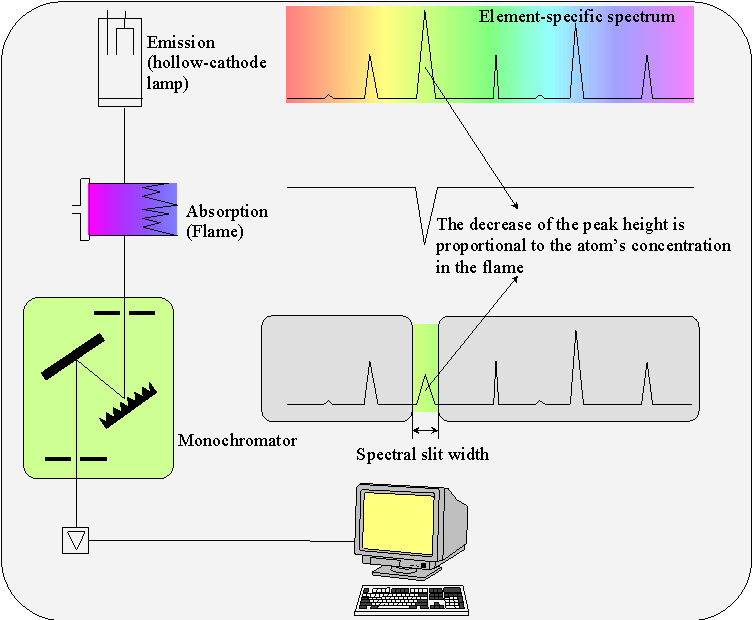
The atomic absorption spectrometry (AAS) is an analytical method based on the absorption of UV or visible light by gaseous atoms. The sample becomes atomized by injecting a solution into a flame (flame AAS) or by heating a dried sample in an energized graphite pipe (graphite tube AAS). As source of light, a hollow-cathode lamp (HCL) is used to determine the contained element. These element’s atoms in the flame absorb precisely the wavelength sent by the source of light. The absorption is proportional to the concentration. The light produced by the HCL is spectrally divided in the monochromator, and only the resonance line is passed trough the exit slit.
with :
- E - extinction,
- T - light transmission expressed as a percentage (transmission),
- ID - intensity of the transmitted signal,
- I0 - intensity of the original signal,
- e - extinction coefficient (proportionality factor),
- c – concentration
- l - layer thickness (= distance traversed by the light ray through the flame/atomic cloud).
Determination of the exchanged Fe-ions in the filtrate (1) with AAS
- Flame atomic absorption spectrometer
- 25 measuring flasks of 100 ml
- Measuring pipettes
- Fe stock solution I (1000 mg/L)
- 20% CaCl2 solution
- Matrix solution :
- transfer 250 mL of the NH4Cl extraction solution in a 1000 mL measuring flask and fill up to the mark with distillate water
Determination of Fe
Interferences (disturbances)
The Fe signal can be reduced by silicates. This disturbance can be minimized by an addition of a 0.2% CaCl2 solution.
Execution
Prepare 100 mL of stock solution II with a concentration of 100 mg Fe/L.
Preparation of the Fe calibration solutions
Prepare the matrix solutions with following concentrations in 100 mL measuring flask.
Add 1 mL of 20% CaCl2 solution to every standard.
| Fe concentration | |
| Blank value | 0 mg/L Fe |
| Calibration solution 1 | 2,0 mg/L Fe |
| Calibration solution 2 | 5,0 mg/L Fe |