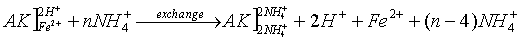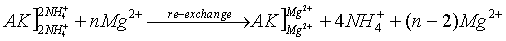Inorganic Analysis
Chemical analysis involves acquisition and interpretation of information about natural systems with the aid of scientific methods. In qualitative analysis, the type and the detection limit of a substance are determined, whereas in quantitative analysis its concentration or its amount in a complex environment (the matrix) are of particular interest.
In inorganic analysis, all known elements and their compounds (except any carbon compounds, but including cyanides, carbides and carbonates) are analyzed.
In order to understand operation of organisms, organism communities, ecosystems, landscapes, but also interactions between them and their biotic and abiotic environment, it is essential to characterize their chemical composition and element budgets. For that reason, quantitative analysis has a predominant position in the inorganic part of this practical course of environmental analysis.
In the first experiments, the amount of exchangeable iron and the effective cation exchange capacity of a soil will be determined. Complementarily, calcium and magnesium in drinking water will be measured.
The following analytical techniques will be introduced to you: spectrophotometry, atomic absorption, volumetry (titration).
Determination of exchangeable iron and the effective cation exchange capacity (CECeff)
Conceptual formulation of the experiment
- exchange of adsorbed cations with NH4+ and re-exchange with Mg2+
- determination of iron in the soil solution (filtrate 1) using atomic absorption
- determination of NH4+ in the soil solution (filtrate 2) using spectrophotometry
- calculation of the amount of exchangeable iron and CECeff
The basic idea of the determination of CEC is the occupation of vacancies and the replacement of adsorbed cations (Fe and others) with an easily exchangeable specific cation from a high concentrated solution (e.g. NH4+). The cation exchange capacity is the capability of soils to adsorb a certain amount of exchangeable cations. The CEC has the unit cmolc/kg soil (centimolar amount of charges per kg soil).
The CEC of a soil represents the proportion of colloid and humic matter, with negative charges and, thus, capable for cation exchange. At pH 7-8 (e.g. with presence of lime or with buffered solutions), the CEC is declared as the potential cation exchange capacity (CECpot); at the actual pH of the soil, it is stated to be the effective cation exchange capacity (CECeff).
The exchangeable amount of ions can be determined using the following procedure:
4.
Mg2+-solution is transferred to the NH4+ saturated soil, the released NH4+ by ion exchange to this solution are determined spectrophotometrically.
(AK – soil: the bracket "]" symbolizes a negatively charged surface with cations adsorbed to it)