Volumetry
Determination of drinking water total hardness
Calcium and magnesium occur in all natural waters. The water hardness is its ability to precipitate calcium and magnesium salts of fatty acids. In water technology both elements play a substantial role with the formation of protective layers in the pipe system.
Titration is an analytical technique for determining the concentrations of analytes and to investigate the amount of chemicals involved in reactions. Titrations are widely used because they are quick, convenient and easy to automate.
The procedure only gives accurate results if the reaction is rapid and exactly as described by the chemical equation. So long as these conditions apply, titrations can be used to study acid-base, redox, precipitation and complex-forming reactions.
A defined volume of the unknown is transferred to a flask with a pipette, then the standard solution is added carefully from a burette. The end-point is determined by adding a few drops of an indicator or by using an instrument such as a pH meter, a colorimeter or a conductivity meter.
Complex-forming titration is a practical technique used to determine the concentration of metal ions. In a titration the volume of a standard solution of a complex-forming reagent needed to react exactly with the metal ions in a defined volume of the unknown solution is measured.
Complex-forming titrations often use a standard solution of EDTA which forms very stable complexes with metal ions.
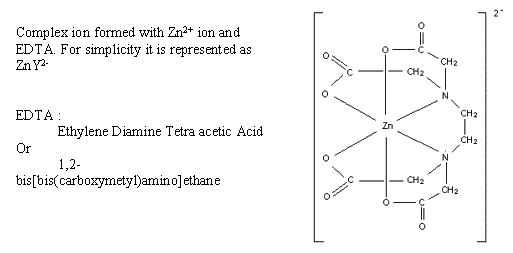
To find the end-point the analyst adds an indicator which forms a coloured but less stable complex with the metal ion. Additionally, a buffer solution is added to establish a suitable pH value. A suitable indicator is Eriochrome black T which is blue in a solution at pH 10. At the start of the titration it produces a wine-red complex. EDTA forms a more stable complex with the metal ions and so takes them away from the indicator. At the end point all the metal ions have been complexed with EDTA. The last drop of EDTA leaves no metal ions to form the red complex with the indicator so the indicator turns blue again.
Before doing the experiment, we have to be sure that only Ca and Mg would react with EDTA, and that we don’t titrate other ions.
With an ion-chromatograph, we are able to analyze the composition of drinking water.
For example, analyses shows for our tap water:
| 78 mg/L Ca | 80 mg/L SO4 |
| 13 mg/L Mg | 25 mg/L Cl |
| 12 mg/L Na | 2 mg/L NO3 |
| 2 mg/L K | 1 mg/L NO2 |
After a quick look to a complex constant table, it is obvious that the following reactions are the main reactions of the titration:
Ca2+ + Y4- à CaY2- Kf (stability constant)= 1010.7
Mg2+ + Y4- à MgY2+ Kf (stability constant) = 108.7
Both reaction are complete and quick. Thus, the use complex-forming titration is justified.
Volumetric determination of drinking water total hardness
Materials
- Titration materials:
- 1 burette
- Erlenmeyer flasks
- 1 shaker
- EDTA solution 0.01 mol/L:
- Dissolve 3.725 g Ethylene Diamine Tetra acetic Acid disodium salt dihydrate in a 1 L measuring flask .
- Buffer solution pH=10:
- Dissolve 6.75 g ammonium chloride and 0.05 g Ethylene Diamine Tetra acetic Acid disodium magnesium salt in a 100 mL measuring flask with 57 mL of a 25% NH4OH solution
- Indicator solution:
- Dissolve 0.5 g Eriochrome black T in a 100 mL triethanolamine solution
Execution
- Pour 100 mL of the sample, 4 mL of buffer solution and 3 drops of indicator solution in a flask
- For the first time, titrate rapidly with the EDTA solution until observing the colour changing
- With a second and a third titration go immediately to approximately 0.5 mL fewer than with the first one. Titrate slowly and precisely!
Analysis
The total concentration of Ca2+ and Mg2+ Ions in the sample at the equilibrium:
nCa2+ + Mg2+=nEDTA
[Ca2+ + Mg2+]=VEDTA / Vsample*[EDTA]
with :
- VEDTA as volume of EDTA solution used in mL,
- Vsample as sample’s volume in mL
- [EDTA] as concentration of the EDTA solution in mmol/L ( here : 10 mmol/L )
The results is indicated in mmol/L. 1 mmol/L (Ca2++Mg2+) corresponds to 5.608 °dH (degrees of German hardness).
Water classification seeing the gradation of the water hardness
| Gradation | °dH |
|---|---|
| very soft | 0 ... 4 |
| soft | 4 ... 8 |
| middle hard | 8 ... 17 |
| hard | 17 ... 28 |
| very hard | 28 ... |
Calculate the concentration of Ca2+ + Mg2+.
To finish with, calculate the total hardness of the water.