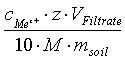UV-VIS-Spectroscopy
Determination of Ammonium (NH4+-Ion)
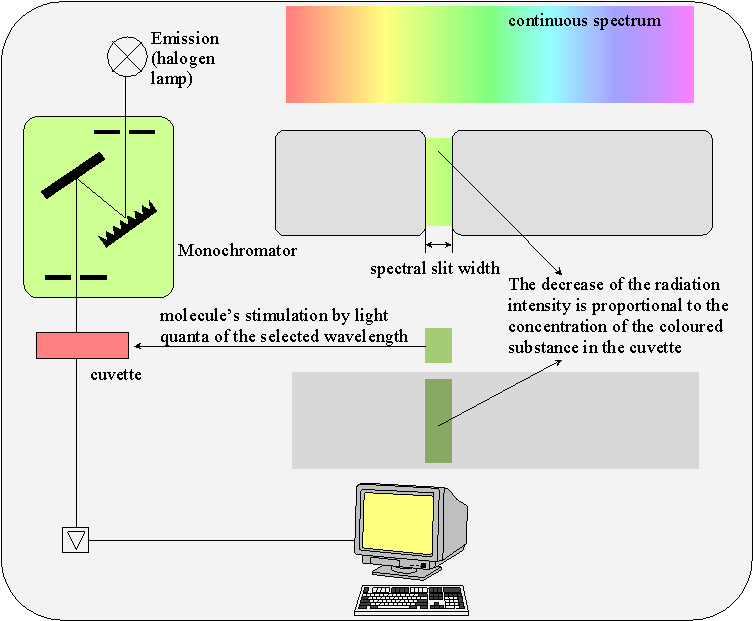
A UV-VIS spectrometer (UV for ultraviolet, VIS for visible = visible light; l = 380-750 nm) measures the light’s absorption or transmission. It always consists of a light source, lenses and slits for beam arrangement, a monochromator for generation of monochromatic light, a cuvette with sample solution and a photodetector.
In a single beam photometer (e.g. Spekol 11), first the absorption of the blank solution will be compensated to zero and then, the sample solution will be measured.
In double beam photometers (e.g. PE Lambda), the light beam simultaneously passes the reference cuvette (blank solution) and the sample cuvette.
The determination of concentration with a UV-VIS spectrometer is based on the Lambert-Beer law.
Determination of re-exchanged NH4+-Ions in the filtrate (2) with UV-VIS spectroscopy
Material
- Spectrophotometer and 1 cm cuvettes
- 12 x 50 mL measuring flasks
- Disodium pentacyanonitrosylferrate- sodium salicylate solution:
- Dissolve 200 mg sodium nitroprusside, Na2[Fe(CN)5NO].2 H2O (Toxic R: 25; S: 22-37-45) and 34,0 g of sodium salicylate (harmful; R: 22; S: 24/25) in a 200 mL measuring flask in approximately 150 mL of distillate water.
- Then, fill up with distillate water.
- The solution is perishable.
- Citrate solution:
- Dissolve 28 g of citric acid monohydrate in a 200 mL measuring flask filled with 120 mL of distillate water.
- Add by portions 20 g of NaOH (corrosive; R: 35; S: 26-37/39-45) (caution, strong heat development!) and after cooling, fill up to the mark on the measuring flask with distillate water.
- Dichlorine isocyanuric acid solution:
- Dissolve 60 mg of dichlorine isocyanuric acid sodium salt dihydrate (irritant, harmful; R. 22-31 36/37; S: 8-26-41), in 10 mL demineralized water.
- The solution must be prepared daily.
- Oxidation reagent:
- Mix before use 4 portions of citrate solution with 1 portion of dichlorine isocyanuric acid solution.
- NH4+ standard solution (0.1 mg NH4+/mL)
Preparation of the NH4+ calibration solution
- Pour 40 mL of 0.2 N MgCl2 solution in each of the 50 mL measuring flasks and the following quantities of ammonium standard solution with a pipette:
| Quantity of NH4+ standard in µL | Equivalent to mg NH4+/L in 40 mL of Filtrate (2) | |
|---|---|---|
| Blank value | 0 | 0 |
| Standard 1 | 200 | 0.50 |
| Standard 2 | 400 | 1.00 |
| Standard 3 | 600 | 1.50 |
| Standard 4 | 800 | 2.00 |
| Standard 5 | 1000 | 2.50 |
Determination of the NH4+ concentration in the filtrate (2)
- Pipette 40.0 mL of filtrate (2) in a 50 mL measuring flasks, then add 2,0 mL Disodium pentacyanonitrosylferrate sodium salicylate solution and 3.0 mL of oxidation reagent. Fill up with distilled water and shake several times.
- After 90 min, measure the extinction at 690,0 nm and a layer thickness of 1 cm. The determination of the NH4+-concentration is based on calibration curves.
With :
- cMeZ+ cation concentration in the filtrate in mg/L,
- z - electrovalence,
- VFiltrate – filtrate’s volume in mL,
- M - molar mass in g/mol
- msoil - quantity of soil originally weighted in g,
- 10 - conversion factor from mmolc to cmolc.